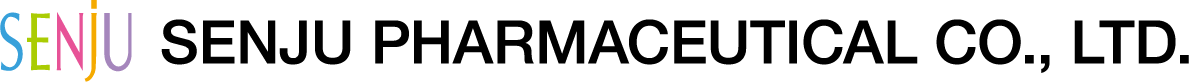Category 2 OTC medicines
Mytear® V
Resolves redness of the eye and provides healthy and clear eyes.
CHARACTERISTICS
- Compounded with "tetrahydrozoline hydrochloride", a component to relieve redness of the conjunctiva.
- Has effects on hyperemia due to long-time use of a smartphone/tablet computer, lack of sleep, and long-time exposure to ultraviolet light.
- This is an eye drop with mild sensation.
PRECAUTIONS FOR USE
Who should seek consultation
1. The following persons should consult a physician, pharmacist, or registered salesperson* before using this medicine:
- Persons under treatment by a physician
- Persons who have had allergies to medicines or something
- Persons who have suffered from the following symptom: Severe eye pain
- Persons diagnosed with the following condition: Glaucoma
2. Stop using this medicine immediately and consult a physician, pharmacist, or registered salesperson if the following symptoms appear after using this medicine because they might be side effects of the medicine. Take this leaflet with you.
| Affected area | Symptoms |
|---|---|
| Skin | Rashes, redness, itching |
| Eye | Hyperemia, itching, swelling |
3. Stop using this medicine immediately and consult a physician, pharmacist, or registered salesperson in the following cases. Take this leaflet with you.
- If there is no improvement in your blurred vision.
- If there is no improvement in your symptoms after using this medicine for 5 to 6 days.
INDICATIONS
Redness of the conjunctiva, eyestrain, prevention of eye troubles (after swimming, or to wash out sweat or dust etc.), foreign-body feeling by hard contact lenses, blurred vision (eye mucus),
DOSAGE AND DIRECTIONS
Apply 1 to 2 drops at a time, 3 to 4 times a day.
< Precautions regarding dosage and directions >
-
- Excessive use of this medicine may cause abnormal brightness or even hyperemia.
- Children are permitted to use this medicine only under the direction and supervision of a parent or other responsible adult.
- Do not touch the tip of the bottle to your eye, eyelid, or eye lashes. (If eye mucus or other foreign matter, etc. is mixed in the bottle, the medical solution can be contaminated or clouded.) In addition, do not use a clouded solution.
- Do not use this medicine while wearing soft contact lenses.
- For ophthalmic use only.
- Please follow the recommended dosage and directions.
-
INGREDIENTS
| INGREDIENTS | CONTENTS(in 1mL) |
|---|---|
| Tetrahydrozoline hydrochloride | 0.5 mg |
Inactive ingredients
Sodium chloride, boric acid, sodium borate, disodium edetate hydrate, benzalkonium chloride, pH control chemicals
PRECAUTIONS FOR STORAGE AND HANDLING
-
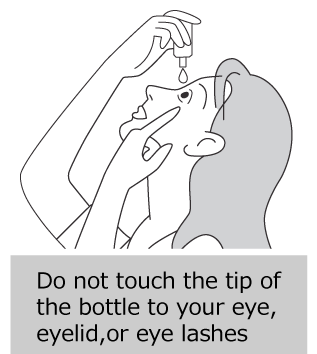
- Keep the bottle tightly closed and store it in a cool place away from direct sunlight. Do not leave the medicine where the temperature might become high (especially in cars or near heating appliances).
- Keep out of the reach of children.
- To prevent improper use or loss of quality, do not transfer the medicine to another container.
- Do not transfer other things to the bottle.
- Do not share the medicine with others.
- Do not use this medicine after the expiration date. Even before the expiration date, use this medicine as soon as possible once the inner pouch is opened.
- Depending on the conditions of storage, ingredients of the medical solution can be crystalized and attached around the tip of the bottle or inside the cap. In such a case, wipe lightly with clean gauze before use.
-
| PACKAGE | 15mL |
|---|